|
ARTE E CIÊNCIA
Art Conservation and Nanotechnology: A wonderful confluence of Arts and Sciences. Art is a language that can transcend geographical, cultural, and chronological boundaries, and helps us explore the legacy of our shared humanity and creativity. Thomas P. Campbell, the 9th director of The Metropolitan Institute of Art, said it well in his TED talk: Bringing people face-to-face with our objects is a way of bringing them face-to-face with people across time, across space, whose lives may have been very different to our own, but who, like us, had hopes and dreams, frustrations and achievements in their lives, and I think this is a process that helps us better understand ourselves, helps us make better decisions about where we are going. The use of art as a means of connecting people is something that is increasingly relevant in today’s diverse and complicated world, making the conservation and preservation of art all the more important. Art conservation unites the fields of arts and sciences, in the pursuit of salvaging centuries old artworks, as well as protecting them from possible future damage. But what does this have to do with nanotechnology?  The conservation science lab at the Indianapolis Museum of Art Image by Richard McCoy
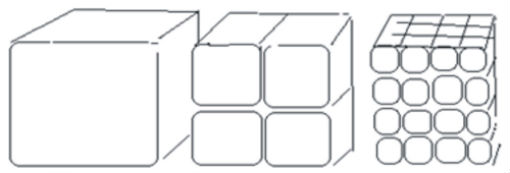 An example of how smaller particles (right) have more surface area than bigger particles (left). Solvent on the surface of nanodroplets will have more surface area to interact with the dirt on a painting than conventional solvent materials. Image by Julie Sandeen
 Paintings in the Brancacci Chapel Image by Sailko
To repair some damage caused by these problematic synthetic polymers, restorers used a water-based microemulsion system containing water and chemicals called esters along with surfactants. They successfully removed old acrylic–vinyl copolymers that had been applied to Maya and Nahua murals in the archaeological sites of Mayapan and Cholula in Mexico.2 The nanosized containers of the organic esters are very effective in interacting with the flaking polymer coating, and detaching them from the surface. This system was also successfully used in removing a commercial polymer coating that had been applied in the 1970s to preserve the paintings of the Annunciation Basilica in Nazareth (Israel) depicting the Garden of Eden.  Example of painting restoration from the Annunciation Basilica in Nazareth Image adapted with permission from Baglioni et al.4 Copyright 2012 American Chemical Society
 Restoration of Mayan wall paintings in the UNESCO world heritage site of Calakmul, Mexico using Ca(OH)2 nanoparticles. (a) The wall paintings after restoration. (b) Visible light image showing the detaching paint flakes before the application of nanoparticles. (c) Scanning electron microscopy image of the Ca(OH)2 nanoparticles applied to the degraded painted surface. (d) Visible light image showing re-attached and re-adhered paint flakes after the application of nanoparticles. Reprinted by permission from Macmillan Publishers Ltd: Nature Nanotechnology, copyright 20151
Another example of restoring hard surfaces uses a different type of nanoparticles. An older method for restoring stone-based structures like historic monuments, churches and cathedrals uses ethyl orthosilicate solutions, which unfortunately can cause cracking in the materials when they dry. A new alternate method involves adding silica nanoparticles to the ethyl orthosilicate solutions, which can form flexible chain structures that reduce the chances of developing cracks1.
 The Cathedral of Konstanz – one example of a structure that had been repaired using ethyl orthosilicate solutions6 Image by Zairon
Journal of Chemical Education: ZnO-Based Sunscreen: The Perfect Example To Introduce Nanoparticles in an Undergraduate or High School Chemistry Lab by Guedens et al. (introduces microemulsions; may require subscription). 2. Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle , Microemulsions , and Gels for the Conservation of Cultural Heritage. Adv. Colloid Interface Sci.2014, 205, 361–371. doi: 10.1016/j.cis.2013.09.008; 3. Baglioni, M.; Giorgi, R.; Berti, D.; Baglioni, P. Smart Cleaning of Cultural Heritage: A New Challenge for Soft Nanoscience. Nanoscale 2012, 4, 42–53. doi: 10.1039/C1NR10911A; 4. Baglioni, M.; Berti, D.; Teixeira, J.; Giorgi, R.; Baglioni, P. Nanostructured Surfactant-Based Systems for the Removal of Polymers from Wall Paintings: A Small-Angle Neutron Scattering Study. Langmuir 2012, 28, 15193-15202. doi: 10.1021/la303463m 5. Baglioni, P., Carrasco Vargas, R., Cordeiro Baqueiro, M. & Chelazzi, D. in Science and Art: The Painted Surface (eds Sgamellotti, A., Giovanni, B. G. & Miliani, C.) Ch. 4, 68–93 (Royal Society of Chemistry, 2014); 6. Wheeler, G. & Getty Conservation Institute. Alkoxysilanes and the Consolidation of Stone. Los Angeles: Getty Publications, 2005. Center for Sustainable Nanotechnology. Accessed: Feb 10, 2019. |
||||||||||||||||||||||||