|
ARTE E CIÊNCIA
Chemistry and Art in the Mountains of Morocco. Last month, I went on a dream trip – a 9-day tour through Morocco. Morocco is a beautiful country at the cultural crossroads of Africa and the Middle East, with a touch of Europe (it was a French colony for much of the 20thCentury). We visited big cities like Casablanca, Fez, and Marrakech, but my favorite parts of the tour were when we visited the dunes in the Sahara Desert and villages in the Atlas Mountains. It was in one of these mountain villages, specifically Aït Ben Haddou, that I found an exciting example of sustainable chemistry.  Dye vats for the tanneries in Fez; I actually visited a shop that had a view of this work area. They use natural pigments for most of this color work similar to the paints I saw in Aït Ben Haddou. (image by Ben Ostrower)
 The artist’s paints in Aït Ben Haddou (photo by Christy Haynes)
 The artist using heat to bring out the color from his tea-based paint. (photo by Christy Haynes) When I returned from the trip, I did some follow-up reading to understand the chemistry behind these natural pigments. The molecule responsible for the bright yellow color of saffron is called crocin, and its molecular structure looks like this:  Crocin molecule (image by Edgar181)
In addition to its bright yellow color and double bond molecular structure, another important thing about crocin is that it has two sugar molecules stuck on to the ends of its structure (as indicated by the orange arrows). This makes the molecule water soluble, which is why the artisan I met can just put saffron in water and get a nice pigment. The molecule responsible for the blue color of indigo is this one:
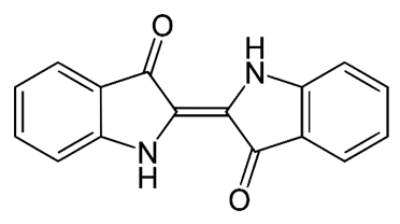 Indigo molecule (image by Yikrazuul)
The chemistry behind the final brown/black color is slightly more complicated. Green tea contains a lot of different molecules, and these specific molecules vary based on the type of green tea used. I did not quiz the Moroccan artisan about his sources of green tea, so I can only make some educated guesses about the chemistry behind this pigment. It seems likely that one critical component of the eventual brown/black color is a molecule known as catechin:
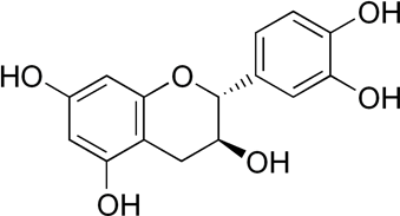 Catechin molecule (image by Edgar181)
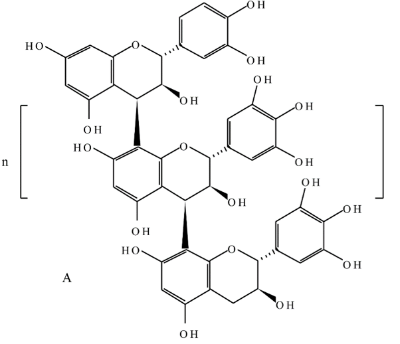 Polymerized catechin (image from Ncube & Van Staden (2015)1
Overall, I love the idea of using naturally occurring, sustainable materials wherever we can. Ancient civilizations clearly knew how to do that, and I really enjoyed seeing how a modern day artist used chemistry to make art that I can hang in my office back in Minnesota to remind me of my travels.  My chemical-artistic souvenir from Morocco (photo by Christy Haynes)
· Blatti, J. Colorful and Creative Chemistry: Making Simple Sustainable Paints with Natural Pigments and Binders. Journal of Chemical Education, 2017, 94 (2), pp 211–215, Doi: 10.1021/acs.jchemed.6b00591; · Royal Society of Chemistry: Making Paint with Minerals lesson plan. 1. Ncube, B. & Van Staden, J. Tilting Plant Metabolism for Improved Metabolite Biosynthesis and Enhanced Human Benefit. Molecules, 2015, 20 (7), 12698-12731, Doi: 3390/molecules200712698. By Christy Haynes. Sustainable Nano. Posted: Jan 30, 2019.
|
||||||||||||||||||||||||