|
NOVIDADES
Pesquisadores descobrem que nanopartículas de ouro podem se dissolver quando interagem com plantas aquáticas de água doce - em vez de permanecerem estáveis como comumente se pensa. When you purchase anything from makeup to paint to sunscreen, chances are it contains engineered nanoparticles. These nanoscale materials have properties that are revolutionizing products—from medicine to agriculture to electronics. But eventually, those nanoparticles will reach natural environments. To use them safely and to their fullest potential, we need to know how they behave in real environments—and if that behavior leads to any unintended consequences. Greg Lowry, professor of civil and environmental engineering at Carnegie Mellon University, studies how nanoparticles behave in and impact the environment. One way researchers have studied nanoparticle fate is by tracking gold nanoparticles—because they are stable and easy to find, or so researchers thought.  The mesocosms at the Center for Environmental Implications of NanoTechnology at Duke University.
The study did not measure toxicity so this doesn’t mean gold nanoparticles are harmful—instead, by better understanding their behavior in biologically active environments, scientists can ultimately use this knowledge to design better nanomaterials. Their findings weree published in Nature Nanotechnology ("Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome"). “This study has opened our eyes to the importance of plants and the plant microbiome in determining the fate of engineered nanomaterials in freshwater environments,” said Lowry. “These plants, and biofilms in general, are important sinks for nanomaterials and are a fascinating compartment to study.” The team looked at exactly what causes this transformation and how quickly it occurs. They conducted their tests in what is called a mesocosm—a controlled natural freshwater environment. The mesocosm, housed at the Center for Environmental Implications of NanoTechnology at Duke University, contains soil, sediment, water, plants, insects, fish, and microorganisms that ordinarily live in these natural environments. Avellan and the research team released gold nanoparticles into the mesocosm water in very low amounts every week to mimic long-term, low dose inputs expected from nanomaterial uses. They wanted to see how the nanoparticles would behave in a complex, biologically active ecosystem. After six months they found that 70% of the gold was accumulating with the aquatic plants, and that all of the gold nanoparticles had dissolved and changed to other forms of gold. When they took a closer look at the biofilm, or a sticky substance made up of bacteria and microorganisms found on plants, they found that the microorganisms released cyanide that was interacting with the gold nanoparticles. The gold nanoparticles dissolved (or ionized) and formed gold-cyanide along with other gold complexes that remained with the plants. 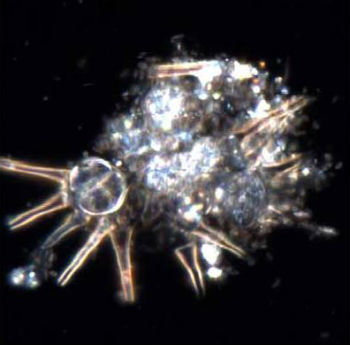 Microscopy image showing the microorganisms present in the biofilm found on subaquatic plants that cause the gold to dissolve.
“We found that gold was accumulating like crazy in the aquatic plants, which was not what we were expecting,” said Astrid Avellan. “So we dug into that and found that gold was associated with these plants, but it wasn’t nanoparticulate anymore.” This is a major breakthrough because gold nanoparticles were thought to be a stable material, and have often been used as a tracer to understand how nanomaterials behave—if you find the nanoparticles then you know where the nanoparticles accumulate. The findings from this paper imply that even relatively inert metal nanoparticles like gold can actually dissolve when they interact with biofilm in water environments.
“The interactions of nanomaterials with the phytobiome can potentially be leveraged to benefit agriculture,” said Lowry. “The research community is only beginning to understand the role of the phtyobiome on plant productivity. This study indicates the potential to design nanomaterials that work together with the phytobiome to improve plant productivity. Successful interventions in agriculture will need to consider how to work synergistically with nature.” Though the effects of the gold transformation need to be studied more, it is possible that it could be toxic to some organisms. The ions could also move faster and farther away than the nanoparticles, distributing differently in organisms and in the environment. The good news is that now researchers have discovered how and why they dissolve, so we can be smart about future uses and applications of nanoparticles—even leveraging this phenomenon for our benefit. “Now we know why and in what conditions gold nanoparticles dissolve,” said Avellan. “So we can take this knowledge and use it to ouradvantage to design better materials. Carnegie Mellon University. Posted: Aug 15, 2018. Assuntos Conexos: |
|||||||||||||||||||||||||