|
NOVIDADES
Out of the box, crystalline MOFs (metal-organic frameworks) look like ordinary salt crystals. But MOFs are anything but ordinary crystals – deep within each crystalline “grain” lies an intricate network of thin, molecular cages that can pull harmful gas emissions like carbon dioxide from the air, and contain them for a really long time. But what if you could design a dual-purpose MOF material that could store carbon dioxide gas molecules for now, and turn them into useful chemicals and fuels for later? Researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have devised a way to do just that – through a self-assembling “superstructure” made of MOFs and nanocrystals. 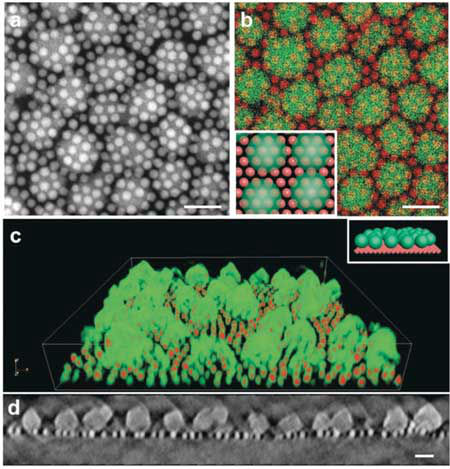 TEM images of a self-assembled nanocrystal-MOF superstructure. Berkeley Lab researchers discovered that iron-oxide nanocrystals and MOFs self-assemble into a ‘sesame-seed ball’ configuration. Image: Jeff Urban et al./Berkeley Lab
For example, one popular method known as X-ray lithography doesn’t work well with MOFs because these porous materials can be easily damaged by an X-ray beam and are challenging to manipulate, said Jeff Urban, the study’s lead author and facility director of Inorganic Nanostructures at Berkeley Lab’s Molecular Foundry, a DOE Office of Science User Facility specializing in nanoscience research. The other problem is that although MOFs and nanocrystals can be mixed in a solution, researchers who have attempted to use methods of self-assembly to combine them have not been able to overcome the natural tendency of these materials to eventually move away from each other – much like the separation you see a few minutes after mixing a homemade salad dressing made of olive oil and vinegar. “Metaphorically, the dense nanocrystal ‘billiard ball’ goes to the bottom, and the less-dense MOF ‘sponge’ floats to the top,” said Urban. Creating a MOF-nanocrystal material that doesn’t separate as oil and water do after being mixed together requires “exquisite control over surface energies, often outside the reach of contemporary synthetic methods,” Urban said. And because they’re not partnering well, MOFs (the material enabling long-term storage and separation) can’t sit next to nanocrystals (the material providing short-term binding and catalysis). Based on their thermodynamics-based calculations, led by Steve Whitelam, a staff scientist at the Molecular Foundry, the Berkeley Lab researchers predicted that the MOF nanoparticles would form a top layer through molecular bonds between the MOFs and nanocrystals. Their simulations, carried out at the National Energy Research Scientific Computing Center (NERSC) – another DOE Office of Science User Facility at Berkeley Lab – also suggested that a formulation of iron-oxide nanocrystals and MOFs would provide the structural uniformity needed to direct the self-assembly process, Urban said. “Before we started this project a few years ago, there weren’t any real guiding principles on how to make MOF-nanocrystal superstructures that would hold up for practical, industrial applications,” Urban said. “These calculations ultimately informed the experiments used to fine-tune the energetics of the self-assembly process. We had enough data predicting that it would work.” The researchers then used a technique known as resonant soft X-ray scattering (RSoXS) at the Advanced Light Source – a DOE Office of Science User Facility that specializes in lower energy, “soft” X-ray light for studying the properties of materials – to confirm the structural order observed in the electron microscopy experiments. In the field of self-assembly, scientists usually expect to see a 2D lattice. “This configuration was so unexpected. It was fascinating – we weren’t aware of any precedent for this phenomenon, but we had to find out why this was occurring.” Urban said that the sesame-seed ball configuration is formed by a reaction between the materials that minimizes the thermodynamic self-energy of the MOF with the self-energy of the iron-oxide nanocrystal. Unlike previous MOF/nanocrystal interactions, the molecular interactions between the MOF and the iron-oxide nanocrystal drive the self-assembly of the two materials without compromising their function. The new design is also the first to loosen rigid requirements for uniform particle sizes of previous self-assembly methods, opening the door for a new MOF design playbook for electronics, optics, catalysis, and biomedicine. Now that they’ve successfully demonstrated the self-assembly of MOFs with catalytic nanocrystals, Urban and his team hope to further customize these superstructures using material combinations targeted for solar energy storage applications, where waste chemicals could be turned into feedstocks for renewable fuels. Berkeley Lab. Posted: Jan 09, 2019. |
|||||||||||||||||||||||||