|
NOVIDADES
For the first time, researchers used benzene - a common hydrocarbon - to create a novel kind of molecular nanotube, which could lead to new nanocarbon-based semiconductor applications (Science, "Finite phenine nanotubes with periodic vacancy defects"). 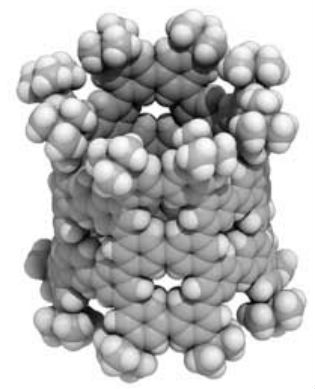 A nanometer-sized pNT cylinder made of 40 benzenes. The cylinder is tens of thousands of times thinner than a human hair. Image: Hiroyuki Isobe
Their phenine nanotube (pNT) is beautiful to see for its pleasing symmetry and simplicity, which is a stark contrast to its complex means of coming into being. Chemical synthesis of nanotubes is notoriously difficult and challenging, even more so if you wish to delicately control the structures in question to provide unique properties and functions. Typical carbon nanotubes are famous for their perfect graphite structures without defects, but they vary widely in length and diameter. Isobe and his team wanted a single type of nanotube, a novel form with controlled defects within its nanometer-sized cylindrical structure allowing for additional molecules to add properties and functions. The researchers’ novel process of synthesis starts with benzene, a hexagonal ring of six carbon atoms. They use reactions to combine six of these benzenes to make a larger hexagonal ring called a cyclo-meta-phenylene (CMP). Platinum atoms are then used which allow four CMPs to form an open-ended cube. When the platinum is removed, the cube springs into a thick circle and this is furnished with bridging molecules on both ends enabling the tube shape. 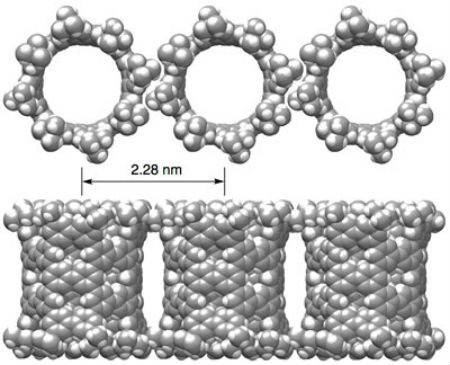 In crystals, pNT molecules are aligned in parallel. Image: Hiroyuki Isobe
“A crystal of pNT is also interesting: The pNT molecules are aligned and packed in a lattice rich with pores and voids,” Isobe explains. “These nanopores can encapsulate various substances which imbue the pNT crystal with properties useful in electronic applications. One molecule we successfully embedded into pNT was a large carbon molecule called fullerene (C70).” “A team lead by Kroto/Curl/Smalley discovered fullerenes in 1985. It is said that Sir Harold Kroto fell in love with the beautiful molecule,” continues Isobe. “We feel the same way about pNT. We were shocked to see the molecular structure from crystallographic analysis. A perfect cylindrical structure with fourfold symmetry emerges from our chemical synthesis.” 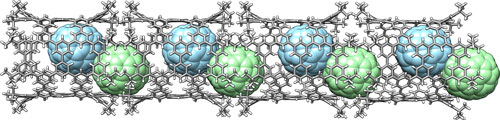 One pNT molecule encapsulates two C70 molecules in its interior. pNT molecules are aligned in a crystal, which results in a linear array of C70 molecules. Image: Hiroyuki Isobe
University of Tokyo. Jan 10, 2019. |
|||||||||||||||||||||||||