|
NOVIDADES
Borophene, the atomically flat form of boron, differs from graphene and other two-dimensional (2D) materials in an important way: It can't be reduced from a larger natural form because bulk boron is not naturally layered. While graphite is composed of stacks of atomically thin sheets that can be peeled off one at a time to make graphene, there is no such analogous process for making 2D boron. Or so researchers had thought. Previous research proposed that 2D sheets of boron cannot form without the support of a substrate, which was believed to have a predominant role in the crystallization of borophene. The theory was that the crystalline order of the substrate controls the crystalline order of the so synthesized borophene. The experimental realization of borophene had been achieved only via atomic layer deposition and molecular beam epitaxy on a silver substrate under ultrahigh vacuum condition (read more: "Synthesis of borophene – atomically thin metallic boron"). This previous work also suggested that there are primarily two planar phases of borophene, namely, β12 and X3, out of which X3 is metastable whereas β12 is higher temperature phase. Now, however, an international team of researchers has synthesized free-standing borophene – β12, X3 and intermediate phases – for the first time and in a scalable manner. As the team reports in Advanced Materials ("Freestanding Borophene and Its Hybrids"), they discovered a facile and scalable liquid-phase synthesis method of freestanding borophene sheets via sonochemical exfoliation. 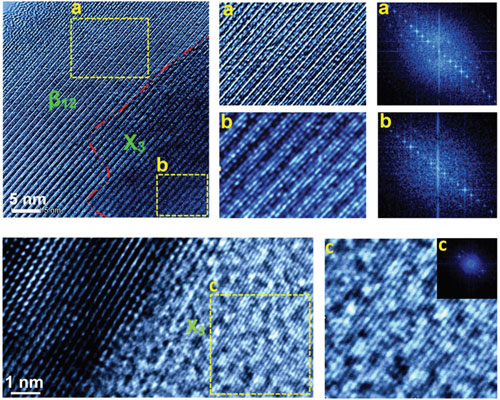 a–c) HRTEM of borophene sheet obtained through sonochemical route in acetone solvent having: a) β12, b) intermediate, and c) X3-like phase along with their FFT patterns, respectively. (Reprinted with permission by Wiley-VCH Verlag
Because borophene in its 2D form is metallic, it holds promise for possible applications in energy storage, catalysis, photovoltaics, and various sensing applications. "Another intriguing feature of borophene is its atomic configuration," Prof. Ajayan Vinu, Director Global Innovative Center for Advanced Nanomaterials (GICAN) at The University of Newcastle in Australia, points out. "Unlike graphene, which is structurally isotropic in nature, the β12 phase of borophene is anisotropic and that in turn is expected to exhibit higher carrier density and mechanical stiffness in preferred direction." In anisotropic materials, their mechanical or electronic properties – like their electrical conductivity – vary with different crystallographic orientations. In contrast, the properties of an isotropic material like graphene are the same in all directions. In their work, Vinu, Prashant, and their collaborators explored freestanding borophene sheets for their potential applications including gas sensing, photosensing, molecular sensing, and strain sensing. In addition, the team synthesized hybrids of freestanding borophene with other 2D materials (graphene and MoS2). "We need to follow up our findings with further detailed exploration of the role of solvents in the exfoliation process and the resulting structural phases," Vinu concludes. "Also, filtering out borophene from the reaction solution is a challenge and we need to overcome that." By Michael Berger. NW. Accessed: May 18, 2019. |
|||||||||||||||||||||||||