|
NOVIDADES
Carbon, one of the most abundant elements in the universe, can exist in different forms (called allotropes) giving it completely different properties from color to shape to hardness. For example, in a diamond every carbon atom is bonded to four neighboring carbons, whereas in graphite, graphene, carbon nanotubes and fullerenes, every carbon atom is bonded to three neighboring carbons. While these are well studied forms of carbon, there are lesser known forms and one in particular has been elusive – cyclocarbons, where the carbon atoms have only two neighbors, arranged in the shape of a ring. Discussed for many years, the structure of cyclocarbons was unknown, and two possibilities were debated, either with all the bonds in the ring of the same length (double bonds only) or with alternating shorter and longer bonds (alternating single and triple bonds). Adding to the drama, evidence for their existence was published in the gas phase, but due to their high reactivity, they could not be isolated and characterized – until now. 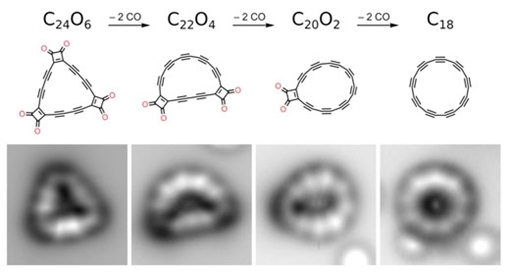 Figure 1: From left to right, precursor molecule C24O6, intermediates C22O4 and C20O2 and the final product cyclo[18]carbon C18 created on surface by dissociating CO masking groups using atom manipulation. The bottom row shows atomic force microscopy (AFM) data using a CO functionalized tip, obtained on bilayer NaCl on a Cu single crystal.
Initially, we focused on linear segments of two-fold coordinated carbons, exploring possible routes for creating such carbon-rich materials by atom manipulation, that is by triggering chemical reactions by applying voltage pulses with the tip of the atomic force microscope. We found that such segments could be formed on a copper substrate covered by a very thin layer of table salt (a bilayer of NaCl). Because the salt layer is chemically very inert, the reactive molecules did not form covalent bonds to it (Nature Chemistry, "Polyyne formation via skeletal rearrangement induced by atomic manipulation"). After the successful creation of the linear carbon segments, we attempted to create cyclocarbon on the same surface. To this end, the Oxford group synthesized a precursor to cyclo[18]carbon (see Figure 1), that is a ring of 18 carbon atoms. This carbon oxide precursor, C24O6, has a triangular shape and in addition to the 18 carbon atoms it contains six carbon monoxide (CO) groups, increasing the stability of the molecule. The synthesis of C18 from C24O6 was first investigated 30 years ago by François Diederich and Yves Rubin, who were then based at the University of California, Los Angeles ("Precursors to the cyclo[n]carbons: [4n + 2]- and [4n]annulenes with unusual stabilities"); now, with recent developments in atomic force microscopy, we can see the product in atomic detail. Lorel Scriven synthesized the carbon oxide, C24O6, in Oxford and took part in the first AFM experiments at IBM Research – Zurich together with the IBM team. Using AFM, we located the precursor molecules, prepared on the thin salt film. Using voltage pulses applied to the tip of the AFM, we could remove pairs of CO-groups from the precursor. We identified intermediates with two and four CO-groups removed. Eventually, we were also able to remove all six CO-groups and to form cyclo[18]carbon. On the cold, inert surface, the molecules are stable enough to facilitate their investigation. In the AFM images, we observed nine bright lobes arranged in a circle, transitioning into corners of a nonagon as we move closer with the probe tip. Comparison with simulations confirmed that the bright lobes and the corners of the nonagon indicate the positions of triple bonds in cyclo[18]carbon. We revealed the polyynic structure of cyclo[18]carbon, that is, we found that the structure is the one with alternating single and triple bonds. Future applications are suggested by the fact that we could fuse cyclocarbons and/or cyclic carbonoxides by atom manipulation. This possibility of forming larger carbon rich structures by fusing molecules with atom manipulation opens the way to create more sophisticated carbon-rich molecules and new carbon allotropes. Eventually, custom-made molecular structures might be used as elements for molecular electronics, based on single electron transfer. IBM. Posted: Aug 20, 2019. |
|||||||||||||||||||||||||