|
NOVIDADES
The identification of new chemical bonds is crucial for the design of new material structures. A team led by Jani Kotakoski at the University of Vienna in Austria and Jannik Meyer at the University of Tübingen in Germany has now found unexpected new configurations of oxygen and nitrogen in graphene. They report their findings, and present direct images of the actual atoms, in a paper in Nature Communications. Life as we know it is based on just a handful of different types of atoms, among them carbon, nitrogen and oxygen. The complexity of life derives from the ability of these elements to connect to each other via chemical bonds to form larger structures. Knowing these possible bonding structures allows scientists both to understand the building blocks of life and to design completely new structures. 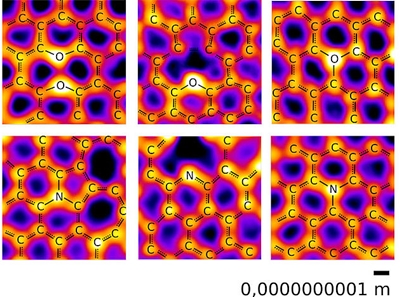 Images of oxygen (upper row) and nitrogen atoms (lower row) in a carbon network, produced at the microscopy laboratory of the University of Vienna. Among the expected configurations, oxygen is also found with three neighbors, as well as in a ‘pair’ configuration with another oxygen. Image: Christoph Hofer and Jannik Meyer
The study revealed that the nitrogen and oxygen atoms bind to their neighbors in a rich variety of configurations. For the most part, it confirmed the textbook picture, which could now be illustrated with direct images of actual atoms: nitrogen atoms were bound to two or three carbon atoms, while most oxygen atoms had two carbon neighbors. "What really surprised us, however, was the additional presence of structures with oxygen bonded to three carbon neighbors," says Christoph Hofer, the lead author of the paper. "Until now, the exception of oxygen with three bonds was only known in an unusual highly charged state, referred to as oxonium, which is difficult to stabilize." In the current study, however, the structures were remarkably stable, allowing them to be imaged by the microscope. The study also revealed a ‘paired oxygen’ configuration, where two oxygen atoms occupy neighboring sites in the graphene lattice but do not create a bond. In addition to providing new insights to the building blocks of life, these new bonding configurations may also lead to the development of new materials. Overall, the study provides a comprehensive overview of the different bonding configurations for nitrogen and oxygen, illustrated directly through images of the individual atoms. While the textbook concept of bonding for carbon, nitrogen and oxygen was mostly confirmed, these common elements can obviously still yield surprises even after decades of study. Materials Today. Accessed: Nov 16, 2019. |
|||||||||||||||||||||||||