|
NOVIDADES
Scientists at Northwestern University, University of California at Santa Barbara, and Argonne National Laboratory determined that the active sites for two reactions involving carbon dioxide (carbon monoxide oxidation and water-gas shift) are associated with small platinum particles and not single platinum atoms as previously proposed. These results directly impact an ongoing debate in the scientific literature regarding the most reactive sites for platinum-based catalysts (Science, "Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts"). 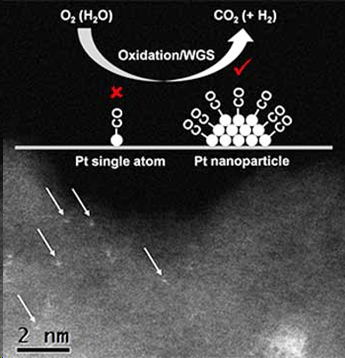 Scientists discovered that for carbon dioxide production (oxidation and water-gas shift (WGS)), the active sites are associated with platinum (Pt) nanoparticles, not single platinum atoms. The aberration-corrected high-angle annular dark-field image shows single platinum atoms in the wet-impregnated 1 wt% Pt/SiO2. (Image: K. Ding and P.C. Stair) Advanced platinum-based catalysts are applicable to a wide array of technologies, ranging from automobile exhaust control systems to hydrogen fuel cells. Knowing how catalytic reactions take place (that is, their chemical mechanisms) is of primary importance for improving their rates, and for the development of new, more efficient catalysts. This study provides insights into noble-metal catalyzed reactions by identifying and characterizing catalytic active sites. Catalysis routinely allows an amazingly large number of chemical reactions to proceed under conditions where such reactions would ordinarily be impossible. As such an important aspect of the energy frontier, improving our understanding of catalysis remains paramount to advancing our nation’s energy future. However, even with such high usage and importance, many catalytic reaction mechanisms remain highly debated or completely shrouded in mystery. One significant debate revolves around the active site structures of platinum and gold: does the active site structure consist of single atoms or small nanoparticles? To answer this question, researchers from Northwestern University, University of California at Santa Barbara, and Argonne National Lab utilized carbon monoxide as a probe molecule while applying advanced infrared (IR) spectroscopy experimental methods. To begin, they distributed an assortment of single platinum atoms and small platinum nanoparticles, “visible” with ultrahigh-resolution transmission electron microscopy, onto a non-reactive support material in various ratios and then exposed the surfaces to carbon monoxide. Due to the energy difference in carbon monoxide binding to a single metal atom versus a small nanoparticle, the scientists detected distinct IR signals for each carbon monoxide bond. Then, they introduced oxygen or water (O2 or H2O) to simulate carbon monoxide oxidation and water-gas shift reactions, respectively, to potentially react with the carbon monoxide bound to the platinum atoms and/or particles. They monitored the extent of the reaction using the IR signals for the various catalytic structures, which provided conclusive results. Only the carbon monoxide molecules adsorbed onto the small platinum nanoparticles reacted with O2 or H2O at low temperatures, showing that the active sites in carbon monoxide oxidation and water-gas shift reactions are associated with the nanoparticles, but not the single atoms. This finding is important not only for platinum and for the carbon monoxide oxidation and water-gas shift reactions, but it can also be applied towards understanding the mechanisms of other noble-metal catalyzed reactions. The knowledge gained from this study could also enable the design of more active and selective catalysts. U.S. Department of Energy. Posted: Nov 23, 2016. |
|||||||||||||||||||||||||