|
NOVIDADES
A team of chemists led by Northwestern University’s William Dichtel has cooked up something big: The scientists created an entirely new type of nanomaterial and watched it form in real time — a chemistry first. “Our work sets the stage for researchers interested in studying the fundamental properties of interesting materials and applied systems, such as solar cells, batteries, sensors, paints and drug delivery systems,” said Dichtel, the Robert L. Letsinger Professor of Chemistry at the Weinberg College of Arts and Sciences. “The findings have enormous implications for how chemists and materials scientists think about nanotechnology and their science in general.” The researchers made covalent organic frameworks (COFs) that are stable — a major advancement. These strong, stiff polymers with an abundance of tiny pores are suitable for storing all kinds of things, including energy, drugs and other cargo. But what limits COFs from realizing these applications is that they are usually prepared as powdery substances that can’t be processed into useful forms. 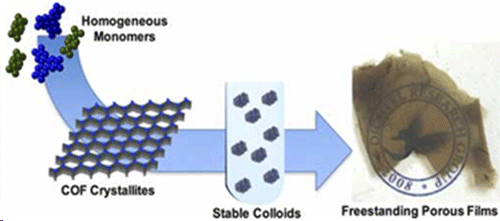 The team reports homogeneous polymerization conditions for boronate ester-linked, 2D COFs that inhibit crystallite precipitation, resulting in stable colloidal suspensions of 2D COF nanoparticles. The hexagonal, layered structures of the colloids are confirmed by small-angle and wide-angle X-ray scattering, and kinetic characterization provides insight into the growth process. (© ACS)
Also, for the first time, the chemists demonstrated that the “cooking,” or heating up, of the ingredients for the nanomaterial can take place inside the imaging tool itself, in this case a powerful microscope called a transmission electron microscope. With this new technique, Dichtel and his team could investigate how molecules come together to form COF colloids. The field of covalent organic frameworks is only a decade old, and much needs to be learned about how the porous polymers form and how to keep them stable. Dichtel is a leader in the young field, focused on bringing unprecedented functionality and improved stability to COFs. The study was published last week in the journal ACS Central Science ("Colloidal Covalent Organic Frameworks"). Dichtel is a corresponding author of the study. The COF colloids are nanoparticles (approximately 50 nanometers in diameter, roughly the size of a virus) made from any number of building blocks in a predictable way. The COF colloids also feature small pores, whose size, shape and chemical groups can be designed precisely. (Each pore is approximately 2.5 nanometers wide, big enough to hold a variety of cargo.) “This is about as close to useful ‘molecular LEGOs’ as I’ve seen,” Dichtel said. “Being able to keep these materials stable in solution is a major step forward towards taking advantage of their unique combination of properties.” For the study, Dichtel teamed up with Nathan C. Gianneschi at the University of California, San Diego, who has developed cutting-edge analysis techniques. Gianneschi, a professor of chemistry and biochemistry, is also a corresponding author of the paper. Dichtel and Gianneschi developed a new way to watch COF colloids form inside a transmission electron microscope, another major advancement. The inability to observe reactions as they occur using electron microscopy has been a major limitation, Gianneschi said. Usually samples have to be dried or frozen to use this technique. The microscopy in this study opens the door to a new dimension: allowing scientists to initiate and observe materials as they form in real time. “This is something that is routine on the macroscale, of course, but has eluded chemists, biologists and physicists at the nanoscale,” Gianneschi said. Megan Fellman, Northwestern University. Posted: Jan 19, 2017. |
|||||||||||||||||||||||||