|
NOVIDADES
Thomas D. Cabot Professor of the Natural Sciences Isaac Silvera and postdoctoral fellow Ranga Dias, have begun to answer some fundamental questions about the nature of matter with the material, which is believed to have a number of applications, including as a room-temperature superconductor. 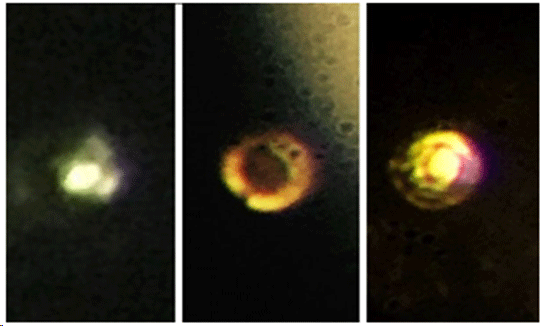 Microscopic images of the stages in the creation of atomic molecular hydrogen: Transparent molecular hydrogen (left) at about 200 GPa, which is converted into black molecular hydrogen, and finally reflective atomic metallic hydrogen at 495 GPa. Credit: Isaac Silvera.
Silvera and Dias were able to squeeze a tiny hydrogen sample at 495 gigapascal (GPa) or more than 71.7 million pounds per square inch, which is greater than the pressure at the center of the Earth. According to Silvera, at such extreme pressures solid molecular hydrogen, which consists of molecules on the lattice sites of the solid, breaks down and the tightly bound molecules dissociate to transform into atomic hydrogen. In the experiment, the two researchers used two small pieces of carefully polished synthetic diamond and treated them to make them even tougher. They then mounted them opposite each other in a device called a diamond anvil cell. “Diamonds are polished with diamond powder and that can gouge out carbon from the surface,” Silvera said. “When we looked at the diamond using atomic force microscopy, we found defects, which could cause it to weaken and break.” They were able to use a reactive ion etching process to shave a tiny layer—just five microns thick—from the diamond’s surface and then coated the diamond with a thin layer of alumina to prevent the hydrogen from diffusing into the crystal structure and embrittling it. Silvera said the discovery could lead to new materials. “One prediction that’s very important is metallic hydrogen is predicted to be meta-stable,” Silvera said. “That means if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure, but remain diamonds when that pressure and heat are removed.” Silvera said understanding whether the material is stable could suggest that metallic hydrogen could act as a superconductor at room temperatures. “As much as 15 percent of energy is lost to dissipation during transmission,” he said, “so if you could make wires from this material and use them in the electrical grid, it could change that story.” Dias explained that a room temperature superconductor could change the transportation system by making magnetic levitation of high-speed trains possible, as well as making electric cars more efficient and improving the performance of many electronic devices. Other applications for the material could provide major improvements in energy production and storage, because superconductors have zero resistance, making superconducting coils more useful to store excess energy, which could then be used whenever it is needed. Another application could be as a more powerful rocket propellant. “It takes a tremendous amount of energy to make metallic hydrogen,” Silvera said. “And if you convert it back to molecular hydrogen, all that energy is released, so that would make it the most powerful rocket propellant known to man, and could revolutionize rocketry.” Fuels are often measured by a specific impulse—a measure in seconds of how fast a propellant is fired from the back of the rocket. The most powerful fuels in use today have a specific impulse of about 450 seconds, while the specific impulse for metallic hydrogen is theorized to be 1,700 seconds. “That would easily allow you to explore the outer planets,” Silvera said. “We would be able to put rockets into orbit with only one stage, versus two and could send up larger payloads, so it could be very important.” The study was published in Science. While the Harvard scientists believe they have made the breakthrough that researchers have been trying to discover for several decades, others aren’t so sure. In a recent article published in Nature, five experts reported doubt on the discovery of metallic hydrogen, saying that the accompanying paper is not convincing. Kenny Walter, RD Magazine. Posted, Jan 27, 2017. |
|||||||||||||||||||||||||