|
NOVIDADES
Single-walled carbon nanotubes act like tiny straws that are so narrow that water confined within cannot freeze into its normal crystal-like structure. In particular, in very thin nanotubes, water molecules align in a single-file manner. At room temperature, each molecule remains orientated in a random direction, creating a disordered chain. For the first time, scientists observed that at a cool 150 K, these molecules go through a quasiphase transition. In this transition, the molecules orient themselves in a highly structured, classically hydrogen-bonded arrangement (Physical Review Letters, "Quasiphase transition in a single file of water molecules encapsulated in (6,5) carbon nanotubes observed by temperature-dependent photoluminescence spectroscopy"). 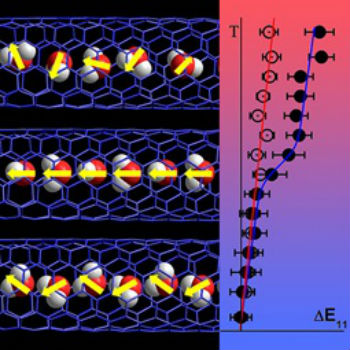 Water molecules (red and white balls) forming a chain in a carbon nanotube (blue lattice) normally orient randomly (yellow arrows; left, top and bottom). As the temperature decreases (right), emission color of an empty nanotube shows a gradual shift (empty data points) whereas a sudden shift in the emission color can be seen at 150 K in the nanotube filled with water (filled data points). This shift is attributed to an unexpected ferroelectric alignment of the water molecules confined within the nanotube (left, middle). Image: Wim Wenseleers, University of Antwerp
While scientists have known that molecules confined within single-walled nanotubes behave differently than their bulk counterparts, it has previously been impossible to study these interactions in a truly uniform environment. For the first time, scientists have been able to select nanotubes of the same chirality and with a very small diameter that can only be filled with one water molecule after the other, yielding a single-file chain. By studying the photoluminescence properties of empty nanotubes compared with water-filled nanotubes, researchers noticed a sudden shift in the emission color of filled nanotubes at ~150 K. Previous studies had observed more general shifts, but scientists could not determine the exact temperature of the shift and could only speculate at the cause behind the shift. In this controlled experiment, where a direct comparison was made between water-filled and empty nanotubes, researchers detected highly ordered structures of water within these nanotubes, a state that previously had only been predicted by theoretical simulations. The group further performed molecular dynamics simulations on this system as a function of temperature. They determined that water dipole orientation is the basis of the phase transition. This finding makes room for more theory to explain the quasiphase transition while the entire study advances the understanding of confined molecules for use in studying complex natural systems and developing novel microfluidic applications. U.S. Department of Energy, Office of Science. Posted: Oct 04, 2017. Assuntos Conexos: |
|||||||||||||||||||||||||