|
NOVIDADES
Most of the time, cooking is a matter of following a recipe - combine specific amounts of specific ingredients in the right way and the predictable outcome is that you'll wind up with a tasty meal. Unfortunately, those same rules don't apply in physics. Despite a deep understanding of the properties of individual atoms - the "ingredients" that make up a crystal - scientists found that, when they are combined they often display new, unanticipated properties, making efforts to design new materials with specific properties little more than guesswork. To make that process more predictable, Harvard Graduate Student Hoi Chun (Adrian) Po Professor of Physics Ashvin Vishwanath and Tokyo university Professor Haruki Watanabe teamed up to produce a system to represent band structures - energy bands, similar to electron orbital, that run through solids - to quickly understand the properties of a given material. The study is described in a recently-published paper in Science Advances ("Structure and topology of band structures in the 1651 magnetic space groups"). 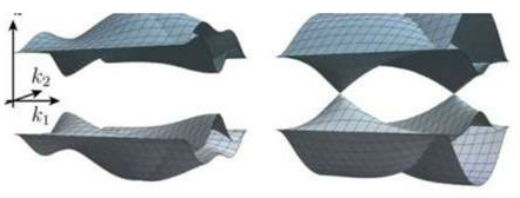 Credit: Science Advances
"A lot of the effort in the early days was on being able to predict whether a material would be an insulator or metallic," he said. "But about 10 or 20 years ago, people realized we could produce these topological materials, (which was exciting) because they have electronic properties that are very desirable. For example, they could be exploited to use the spin of the electron, rather than its charge to perform computation in a more energy efficient way. They may also help create the hardware for a topological quantum computer, one that performs computing in a radically new way. "Insight into band structures would help us find real materials with these topological properties," Vishwanath said. "Right now the way people do this is really more of a guess...and what we are trying to do is to come up with efficient ways of diagnosing whether the material or materials you're interested in have a good chance of having topological properties." But predicting which materials are topological, Vishwanath said, is easier said than done. "The first problem is the huge number of ways in which atoms can form crystals," he said. "Even if you forget about the chemical complexity, forget about which elements are in there, just in the structure...there are 230 ways in which you can put atoms together into crystals." The complexity, though, doesn't end there - Watanabe, Po and Vishwanath, were specifically interested in magnetism, and when that is added to the mix, the number of possible structures jumps dramatically, to 1,651. "So there's a huge complexity there and that's one of the challenges," Vishwanath said. "If we wanted, we could just come up with a long list of options, but that's a very inelegant solution, and doesn't give you any insight into the problem. "We took a different approach," he continued. "The key idea was... we found a way to represent certain key attributes of band structures as a vector in some high-dimensional space". Using that tool, Watanabe, Po and Vishwanath were able to classify all 1,651 possibilities according to whether they were simple insulators, metallic or topological insulators. "While each magnetic space group would previously have taken a graduate student a day to figure out, our new formulation allows for a simple automation of the task which is completed on a laptop for all 1,651 instances in half a day," Po said. Armed with that information, Vishwanath said, researchers can now make more informed choices when designing new materials. "This is a way to narrow down the options," he said. "There are other ways to do it, but we like to think this approach has some advantages." As an example, he pointed to the periodic table, whose organization is designed not just to provide information about various elements, but also about how those elements are related to one another. "You could list all the elements alphabetically, which would make them easy to look up and find," Vishwanath said. "But the periodic table gives you more information. Our system is similar - we can group structures together based on how they're related to each other." "In the literature, there already exists a highly mathematical way (the so-called K-theory) of classifying topological insulators," Watanabe added. "However, this approach hasn't been really used for materials search so far because it requires a high-level of abstract mathematics and is hard to compute. The advantage of our approach is its simplicity - it only involves linear algebra and group theory, both of which are undergraduate math subjects. This means that many researchers in the world can implement the scheme themselves and find candidate magnetic topological materials." Going forward, Vishwanath and colleagues are working closely with materials scientists to use the system to model the expected properties of new materials, and are continuing to explore what information can be teased out of the system. "In some ways, this mirrors our attempts to understand atoms," he said. "What atomic physics did for chemistry was organize things. It explained the periodic table. We are trying to get a similar understanding not for single atoms, but for collections of atoms, and we hope this is one of the organizing principles for that." Harvard University. Posted: Aug 22, 2018. |
|||||||||||||||||||||||||