|
NOVIDADES
Fear is a bad counselor. In the comic book series "Asterix", the Gaul chief Vitalstatistix may be afraid that the sky may fall on his head. In the real world, however, risks should be assessed with a clear mind. To ensure that risk assessments are not carried out emotionally but lead to appropriate decisions, scientists use models to analyze the hazard potential of substances or technologies. Empa researchers are currently investigating the risks of a relatively new class of substances made from tiny materials: drugs manufactured using nanomaterials. It is already known that conventional pharmaceuticals can be released into the environment after being administered or ingested. In the animal world, for example, hormone-like substances can lead to thin-shelled bird eggs, fertility disorders in fish and population declines in otters. 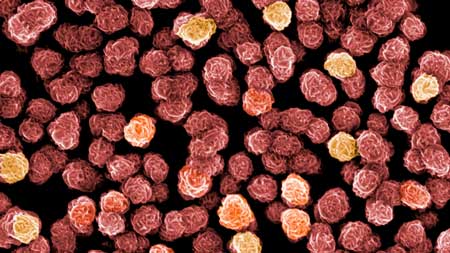 Flower show from Lilliput: silver and iron nanoparticles under the microscope. A "flower" is around 100 nanometers in size. Image: L. Driencourt CSEM / M. Schönenberger Nano Imaging Lab, SNI / University of Basel, colored
Empa researchers led by Bernd Nowack from the "Technology & Society" lab in St. Gallen are currently calculating the risks of these nanomedicines. Among other activities, the team is involved in the international research and innovation project "BIORIMA". The interdisciplinary project develops the risk management of nanobiomaterials for humans and the environment and is funded by "Horizon2020", EU's research and innovation funding program. In order to accurately map the risks of new substances, researchers first determine the threshold value, at which a substance no longer has any harmful effects, as well as the expected amount that is released into the environment. These data are not easy to get hold of, as the fate of the drug in the body and its route to the wastewater treatment plant and from there into rivers and lakes – and thus into the biosphere – must first be determined. Once released into the environment, polymers are altered by biological or physico-chemical decomposition into smaller components. In addition to pharmacological studies, the researchers use analyses of material flows and mathematical environmental models. "For most nanobiomaterials, there are no reliable estimates about the amount of particles being released," says Nowack. These gaps in knowledge must be closed by all means.
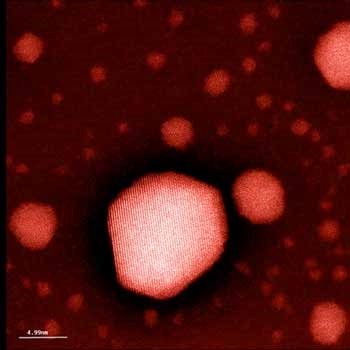 Nanogold: The tiny gold particle in the foreground is about 10 nanometers in diameter. Image: D. Keller / Empa, scanning transmission electron microscope, colored
Some frequently used nanomaterials could now be investigated for the first time on the basis of available data. These include, for example, nano-chitosan, a derivative of a naturally occurring polysaccharide, which is found in the shell of crustaceans and supports wound healing. Other substances under investigation were polyacrylonitrile, PAN for short, which is used in antibacterial therapy, and hydroxyapatite (HAP), a natural mineral that is used in the context of drug release or the regeneration of bone tissue. The analyses showed that chitosan in its conventional form is more toxic to aquatic microorganisms than in its nanoform. The nanopolymer was thus significantly less harmful than conventional drugs that are released into the environment, such as antibiotics or painkillers. The second nanopolymer, PAN, as well as the mineral HAP performed even better. "These substances are virtually non-toxic in water," says Nowack. However, the situation is different for silver nanoparticles, which are used in medicine for their antibacterial effect. In the biosphere, the inorganic nanomaterial exerts the very same toxic effect on microorganisms that are important for the balance in an ecosystem. For complete risk analyses, however, it is necessary to first establish the extent, to which flora and fauna – and ultimately humans – come into contact with these nanomaterials. The Empa team is currently working on these exposure data for the relatively new class of nanomaterials as part of the "BIORIMA" project. The data they obtain are also used in the process of developing new medical products. Empa researcher Claudia Som refers to the "safe by design" approach: "We have developed guidelines for SMEs that allow risky nanobiomaterials to be sorted out early in the costly development process," explains the researcher. Empa's risk analyses thus support sustainable innovation in the field of nanomedicine. H Wigger, B Nowack; Material-specific properties applied to an environmental risk assessment of engineered nanomaterials – implications on grouping and read-across concepts; Nanotoxicology (2019); doi.org: 10.1080/17435390.2019.1568604. Empa. Accessed: Jan 13, 2020. |
|||||||||||||||||||||||||