|
NOVIDADES
The Grignard reaction is used to synthesize carbon-carbon bonds, a crucial step for making new molecules for academic and industry uses. Finding efficient and selective methods for this reaction, using low cost materials and minimal energy resources has been the target of the research activity for more than 100 years. Incredibly enough, the way the Grignard reaction works has been unknown—until now. As we finally understand it, ways to its improvement can now open up. 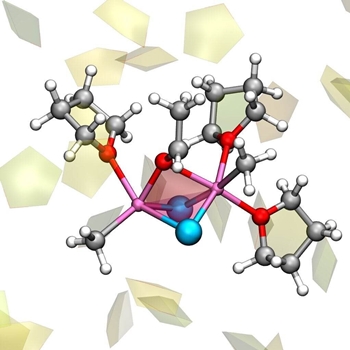 The reactive complex of the Grignard reaction, involving two molecules of Grignard reagent CH3MgCl interacting with acetaldehyde, and with three molecules of tetrahydrofuran aether. Other solvent molecules are drawn as pentagons. Credit: Michele Cascella
It all started 120 years ago with the Grignard reaction that for the first time allowed the tailored formation of carbon-carbon bonds. This reaction has been widely used since then, and thoroughly studied, but never fully understood. Since then, the Grignard reaction has been universally recognized, taught in all basic chemistry courses and extensively used, and still today it shapes the world of organic chemistry. "Not understanding the molecular bases of such a fundamental process is very frustrating for chemists. In fact, such a lack of knowledge prevents scientists from developing ways to optimize the process," says Professor Odile Eisenstein, one of the scientists behind the study. "I guess that the name of Grignard reaction rings a bell in the mind of any chemist. It is probably the first organic chemical reaction I learned about, as a student," says Cascella. Eisenstein and Cascella decided to tackle the problem using computer simulations. Modelling both the reagent and the solvent in a realistic manner, they were able to detect the multiple chemical species during the Schlenk equilibrium. Importantly, their study identified that the whole process is determined by solvent molecules that combine to, or detach from, the magnesium atoms. Thus, the dance of solvent drives the exchange of partners for the magnesium atom, giving rise to the Schlenk equilibrium, and resulting in the different compounds present in the solution. "One of the advantages of a computational study is that you are not restricted by the physical reality, you can systematically test multiple hypotheses, and determine which is the best only a posteriori," says Cascella. By computer simulations accompanied with high-level quantum chemistry data, thanks to a collaboration with Professor Jürgen Gauss (Johannes Gutenberg-University Mainz, Germany), it was possible to establish a series of key points. First, almost all the dancing couples will end up forming stable carbon-carbon bonds, meaning that all the molecules produced by the Schlenk equilibrium promote the formation of carbon-carbon bonds, although at different rates. Second, different partners in the dance request different dancing steps; meaning, different substrate molecules will react following different mechanisms characterised by either heterolytic or homolytic splitting of the magnesium-carbon bond (the two electrons of the bond go to the carbon, or are equally shared between the magnesium and the carbon). "What has always been known as the Grignard reaction is, in reality, a group of reactions that occur simultaneously in the same sample," says Cascella. Their studies demonstrated that unlike other common reactions, in this case the solvent drives the whole chemical process. This was also one of the reasons why the Grignard reaction remained mysterious for so many years: "Systems dominated by the solvent are hard to study, points Eisenstein. Their structure is ever changing, and most experimental methods are not (yet) good enough to see what actually happens. Just like trying to take a photograph of a flock of birds having a shutter speed that is too slow. All you can see in the photo is a blurred mess of feathers and bird-like shapes, but you cannot decide how many birds you have, how they fly, or even which species it is. We cannot determine anything from that. That is where computational methods have an edge." "We have just scratched the surface," says Eisenstein. I"t has long been known that the organometallic reactions can be enhanced with a large variety of additives, such as salts, derivatives of other metal compounds, etc. Additives can make a reaction faster, and cleaner. However, nobody really knows how do they work. Now that we have sufficient understanding on the Grignard reaction, we can construct from this. Once we know how to bake a cake, we can make it tastier and more beautiful. In other words, we can understand the role of additives, and hopefully propose new ones." "For the future, this means that there might be ways to predict improvements for the reaction, with all the implications this will have in places where the synthesis of molecules is needed, such as in medical chemistry and in industry. This reaction is prototypic for a lot of other reactions with metals," says Cascella. "And, unexpectedly, we found that the most reactive species has a very similar shape and structure to the active site of a group of enzymes that are crucial for our existence: the endonucleases." Endonucleases are enzymes that process the DNA in our cells, and they catalyze bond breaking/forming using magnesium as the key cofactor, just like in the Grignard dance. This opens up exciting possibilities for understanding the evolution of these enzymes. It is likely that they started out using less complex, less efficient pathways of the reaction, and then progressively evolved by selecting the most efficient one. On the other hand, designing ligands around the magnesium atoms that mimic the structure of the enzymes could be an excellent route for the improvement of the Grignard reaction itself. As old as it can be, the Grignard reaction confirms today as a great source of inspiration for chemists. Both publications on the Schlenk equilibrium and the Grignard reaction are open access. Explore further Chemists build new chemical structures on unreactive bonds More Information By University of Oslo. Posted: Feb 07, 2020.
|
|||||||||||||||||||||||||