|
NOVIDADES
Diamonds may be forever, but that doesn’t mean they’re the only game in town. Researchers Yasumaru Fujii, Mina Maruyama, and colleagues at the University of Tsukuba have made computer models of a new 3-D carbon structure they call pentadiamond whose properties promise to outshine those of the original material (Phys. Rev. Lett. 2020, DOI: 10.1103/PhysRevLett.125.016001. Unlike a typical diamond, in which every carbon is bonded to four other carbons, a pentadiamond has a mix of carbons bound to three and four other carbons. These form a network of five-membered carbon rings—hence the name pentadiamond. 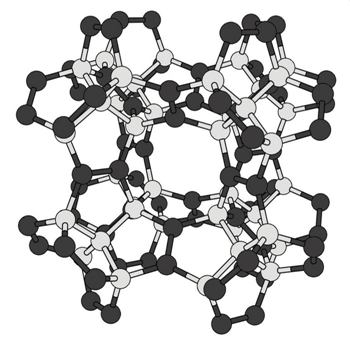 In a pentadiamond, some carbons (black) are bonded to four other atoms, while others (gray) are bonded to three. Credit: Adapted from Physical Review Letters
Chemical & Engineering News. Posted: July 06, 2020.
|
|||||||||||||||||||||||||