|
NOVIDADES
Nanoparticles are increasingly used in cosmetics, food and biomedical applications, e.g. drug delivery and medical imaging. However, the effect of nanoparticles on human health and environment remains poorly understood, and recent reports on nanoparticle toxicity raise safety concerns. We already know that physicochemical properties of nanoparticles alone cannot predict the fate of nanoparticles in biological systems. Current evidence suggests that the formation of a corona on the nanoparticle surface is the most important parameter that controls nanoparticle toxicity. The corona arises from the accumulation of adsorbed biomolecules from blood, saliva, synovial fluid or other fluids in the surroundings. The composition of the corona changes dynamically – remodels – when nanoparticles pass through different tissues. The remodelling of the corona is indirectly related to the intrinsic physiochemical characteristics of nanoparticles, and it depends on the exposure time and type of biomolecules present. 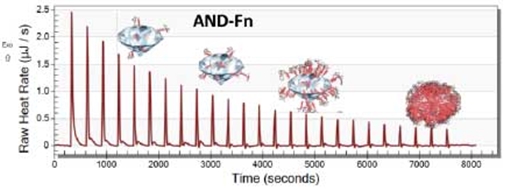 Formation of fibronectin corona on nanodiamond – nano thermal analysis studies. Image: The University of Sydney
The molecular composition of corona can promote or inhibit binding of nanoparticles to cell membrane and consequently modulate the cellular uptake of nanoparticles. Therefore, understanding the role of corona formation on nanoparticle internalisation can lead to the development of more customized nanoparticles. For example, a corona can reduce the adhesion of nanoparticles to the cell membrane to reduce cytotoxicity. However, for nanoparticles which are functionalised with receptor-binding molecules for targeted drug delivery, the formation of corona effectively coats the molecules and consequently deactivates the ability of nanoparticles to recognise cell receptors. While the rapid formation of corona on nanoparticles is known to influence their interactions with cells, there is a gap in fundamental understanding of how corona forms and how it remodels in response to the microenvironment composition and nanoparticle properties. A research team led by researchers from Australia, Singapore and UK investigated how the corona forms and evolves on nanodiamonds and how serum protein corona modulates nanoparticle-cell interactions and toxicity. Reporting their findings in Nanoscale Advances ("Protein corona determines the cytotoxicity of nanodiamonds: implications of corona formation and its remodelling on nanodiamond applications in biomedical imaging and drug delivery"), they showed that protein corona modulates internalisation and cytotoxicity of nanodiamonds in non-phagocytic and phagocytic cells. Specifically, they found that corona made of bovine serum albumin (BSA), which represents the most abundant protein in blood plasma, reduced nanodiamond agglomeration. Fibronectin, the second most abundant protein found in the plasma, exhibited a significantly higher nanodiamond binding affinity than BSA, irrespective of nanodiamond surface charge. Nanodiamonds with BSA corona displayed less cytotoxicity towards nonphagocytic liver cells. However, regardless of the type of corona (fibronectin or BSA), nanodiamonds induced substantial phagocytic cell death. 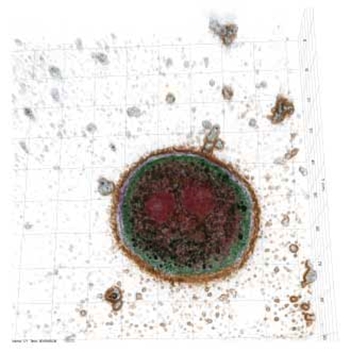 Holotomography image of hepatocytes undergoing apoptosis after internalisation of nanodiamonds (nanodiamonds presented in black). Image: The University of Sydney
"We showed that nanodiamonds induced proinflammatory responses and immune cells were susceptible to secondary endotoxins," the researchers note. "Our results suggest that chronic exposure to nanodiamonds can potentially compromise the effectiveness of immune system. These results emphasise that precise understanding of the corona composition is fundamental to determine the fate of nanoparticles in the body." Through this work the research team demonstrated that the assessment of risks associated with nanoparticles (nanotoxicity) must consider the presence of corona: "It is necessary to integrate studies of corona formation and remodelling into the assessment framework of nanotoxicity." This study also highlights that precise understanding of corona formation and remodelling is likely to unlock new possibilities to manufacture safer nanoparticles for various applications. "For example, nanoparticles with pre-defined coronas can enable the delivery of the therapeutics directly to the target in safe and effective way – personalized treatments – while reducing potential adverse effects of both nanoparticles and therapeutics," the authors conclude. University of Sydney Nano Institute. Posted: September 04, 2020.
|
|||||||||||||||||||||||||