|
NOVIDADES
A recent publication in ACS Nano ("COVID-19 Vaccine Frontrunners and Their Nanotechnology Design") details the contributions that nanotechnology has made to the race of finding a vaccine for the SARS-CoV-2 (COVID-19) virus. Nicole Steinmetz, professor of nanoengineering at UC San Diego and corresponding author on the study, spoke with Nanowerk about the findings in this paper. Steinmetz believes “it is an exciting time for nanotechnology and nanoparticle delivery of nucleic acid-based vaccines and subunit vaccines are poised to make an impact.” The COVID-19 pandemic has left the world reeling following its inception in Wuhan, China. While global efforts are being directed towards a vaccine, thus far none have passed the trial stage. Until humanity achieves herd immunity to this virus, either through exposure and infection or through inoculation, there can be little hope of our returning to our pre-COVID-19 life. Fortunately there are over two hundred academic laboratories and companies around the world searching for a vaccine, and the pace at which they are progressing is unprecedented. One nanotechnology formulation achieved clinical trials a month prior to other conventional approaches. However, due to the stiff regulatory requirements on vaccines to ensure safety, there remain various hurdles that companies must surmount in order to establish a viable vaccine. Nanotechnological approaches have received a boost despite their lack of clinical trials. As an example, mRNA vaccines have been developing for thirty years, but were not previously approved. Due to the adaptability of such technology, prior vaccine candidates can be repurposed using previously developed nanostructures. Nanoparticles and viruses operate on the same scale, and thus there are various nanotechnological aides which are being used in the development of potential vaccines. Nanoparticles are capable of entering the cell through biological channels, and can deliver antigens there. Antigens are typically delivered via either lipid nanoparticles (LNPs), which encapsulate the antigens, or via other benign viruses including Ads. When asked which technologies she thinks holds the most promise in combatting COVID-19, Steinmetz replies “subunit and virus like particle-based vaccines in my view likely will offer the best balance of efficacy and safety.” She thinks “it is an exciting time for nanotechnology in various areas, but I am mostly excited about the push in the vaccine field.” Due to the scale of nanoparticles, they are capable of traveling in vivo differently than other molecules. The lymphatic system, which is critical in orchestrating immune responses, has typically proved challenging to access. However, with the advent of nanoparticles, previously inaccessible pathways have become accessible, with certain trial vaccines attempting to use these to transport antigens. Besides delivering antigens themselves, nanoparticles can also be enlisted to provide adjuvants to cells. Adjuvants effectively catalyze the immune response, allowing the cell to more easily recognize and respond to antigens. Encapsulating both the antigen and adjuvant in the same envelope provides for better targeted delivery and response from the cell. The presence of adjuvants may also reduce the amount of antigen required to engender a response, and thus render a dose-sparing effect. This dose-sparing may drive the cost of immunization down, and render wide-spread inoculations more feasible. Without co-delivery of adjuvants and antigens, antigens are more likely to break down in the body. There are three major nanotechnological techniques of presenting adjuvants and antigens in concert. The first is co-delivery through encapsulation within or conjugation onto a nanoparticle, wherein the adjuvant and antigen are bound within or onto a nanoparticle which is then delivered to the cell. The second is direct antigen-adjuvant conjugation, where the two particles are bonded for delivery. The third approach utilizes the antigen delivery vehicle as an adjuvant itself. 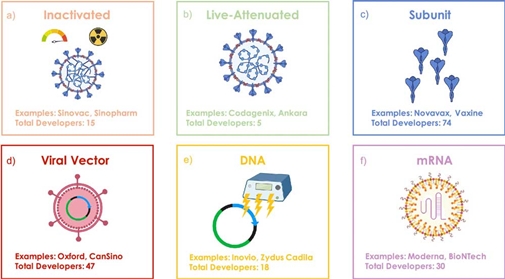 Figure 1. Vaccine types currently under development for SARS-CoV-2. (a) Inactivated vaccine that uses the native virus rendered replication deficient from heat or chemical treatment, (b) live-attenuated vaccine that can replicate, but in a limited manner that cannot cause the disease, (c) subunit vaccine that incorporates subsections of the native virus such as the S protein, (d) viral vector vaccine that encapsulates the genome of a different weakly pathogenic virus with additional DNA that encodes the target viral antigen, (e) DNA vaccine using a DNA plasmid that encodes the target antigen, often administered by electroporation, (f) RNA vaccine of RNA encapsulated within a LNP to decrease RNA degradation and increase translation efficiency. Reprinted with permission by American Chemical Society
Two companies which have been leading the charge to develop COVID-19 vaccines are Moderna and the BioNTech/Pfizer partnership. Both these companies are developing mRNA that encodes subunits of the COVID-19 spike or S protein. Meanwhile, the University of Oxford/Astrazeneca and CanSino are two companies developing vaccines based on non-replicating viral vectors. Viral vectors use DNA rather than mRNA to encode the desired antigens. Both approaches have had limited success as vaccine platforms, and neither is currently approved for use as a vaccine. They both depend on nucleic acids that encode the target antigen, but differ in their delivery. Viral vectors can enter the cell using viral mechanisms, allowing for high-fidelity production of antigens. However, they can also cause immunogenic responses, or cancers if they adhere to the wrong genes. mRNA delivery depends on the use of lipid nanoparticle envelopes to deliver the mRNA cytoplasmically, i.e. directly into the cell. LNP delivery is a more novel technique, which currently cannot match the cellular adhesion efficiency of the long-evolved mechanisms employed by viruses. However, once inside the cell mRNA is capable of being directly translated in the cytoplasm, whereas DNA plasmids must be translated via the nucleus back to the cytoplasm. This allows mRNA to produce more antigens from smaller doses, but DNA tends to produce a longer-lasting antigenic effect. Moderna and BioNTech/Pfizer are both using mRNA vaccines that bind to the S protein site; the S protein being the protein that binds to cells to mediate infection. This is a frequent target as neutralization of the site prevents the virus spreading. There are two targeted components of the S protein, one of which promotes initial adhesion, the next of which promotes fusion with the cell. BioNTech/Pfizer and Moderna have both received promising results from their trials. Neither had sufficient negative effects to warrant the discontinuation of the studies, though certain side effects were recorded. In both cases midlevel doses were preferred. The vaccination trials for Phase III, which will include 30,000 patients each, will not deviate from the rapid schedule developed during Phase II in either case. University of Oxford/Astrazeneca and CanSino are using the Ad viral vector approach. Ads are cold-causing viruses with double-stranded DNA. Cansino has published results from their Phases I and II trials, which showed high doses precipitating a range of side effects, in response to which they are decreasing the concentration of their vaccines. University of Oxford/Astrazeneca only trialled one concentration. Both companies are now continuing trials. Steinmetz says “we have seen many researchers focus on COVID-19; as a result more researchers will continue to apply their nanotechnologies to target infectious diseases - as countermeasures, molecular therapies, vaccines.” She is not sure “whether we have seen ‘novel’ nanotechnologies being developed—what we have seen is that nanotechnology is being pivoted for COVID-19 applications.” Over two hundred COVID-19 vaccine trials are currently underway globally. The urgency of the pandemic has led to research barriers being lifted more rapidly than they otherwise might have, spurring a flood of novel immunization trials. Many of these involve nanotechnology, and only the most prominent have been covered here. As the pandemic progresses we are likely to see further development of these technologies, and potentially an initial vaccine based largely off of novel nanotechnology. A NW exclusive by Jack Seaberry. Jack is a technical writer from the University of Victoria. Posted: Nov 09, 2020.
|
|||||||||||||||||||||||||